A LIPID droplet is a highly dynamic organelle essential for regulating lipid metabolism, storage, and transport. It serves as a specialized compartment for storing lipids synthesized by the cell. Lipid droplets contribute to membrane and organelle stability by buffering toxic lipids, regulating membrane composition, and preventing lipid peroxidation. These functions are vital for cellular survival.
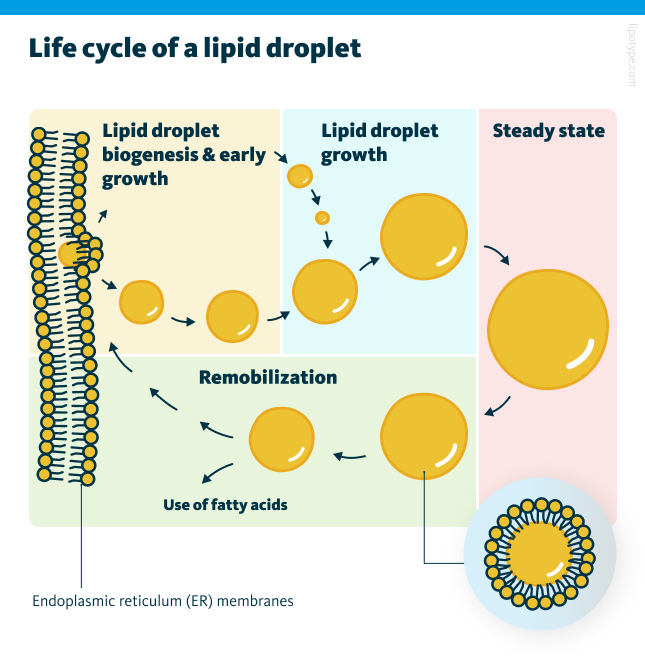
Lipid droplets originate from the endoplasmic reticulum (ER) through localized lipogenesis, particularly under conditions such as nutrient surplus. In this process, fatty acids and sterols are stored within lipid droplets as triacylglycerols (TAGs) and cholesteryl esters. TAG accumulation occurs in the space between the membranes of the ER, leading to the formation of a lipid lens that expands and pushes against the membrane toward the cytosol. As the droplet continues to grow, it eventually buds off into the cytosol. Smaller lipid droplets can either fuse with larger ones or transfer their lipids to them, while larger droplets remain suspended in the cytosol. When lipid remobilization is activated, the stored lipids are broken down. For instance, during starvation, lipid droplets degrade TAGs into free fatty acids and glycerol, which are then utilized for energy production via mitochondrial fatty acid oxidation. Additionally, when new cell membranes need to be synthesized, the breakdown products of TAGs provide essential lipids for phospholipid production and maintenance.
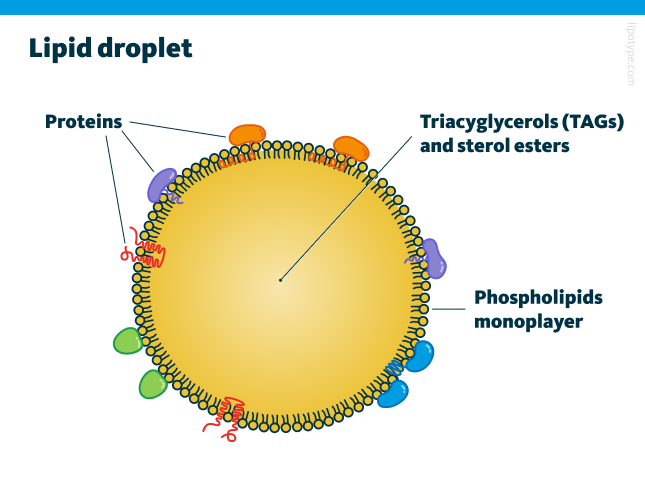
Particular fatty acids can disrupt membrane integrity and interfere with cellular metabolism by increasing membrane permeability through lipotoxicity and oxidative stress. To counteract this, cells sequester these fatty acids within lipid droplets, which are covered by a phospholipid monolayer for protection. In this monolayer, the hydrophobic tails of phospholipids are oriented inward, facing the lipid droplet’s hydrophobic core. Along with fatty acids being stored as TAGs in lipid droplets, other lipids, such as squalene and zeaxanthin, can also accumulate within these structures.
Lipid structural flexibility and lipid droplets
So-Hee Son and colleagues utilized molecular dynamics simulations along with microbial lipid engineering to assess whether lipid structural flexibility influences their migration into lipid droplets. The presence of π bonds in these lipids, whether conjugated or non-conjugated, may affect their selective diffusion into lipid droplets and their subsequent partitioning within the cytosol. To explore this, the researchers examined how lipid flexibility impacts their selective migration into lipid droplets.
The chemical structure analysis of lipids used in both computational and experimental studies revealed that squalene exhibits structural flexibility due to its isolated π bonds. Zeaxanthin on its turn possesses conjugated π bonds, which make it rigid. Flexible lipids can easily deform, which enables them to move through tightly packed TAGs, ultimately influencing lipid droplet composition. Rigid lipids must significantly alter the arrangement of intertwined TAG chains to penetrate deep into a lipid droplet. These rigid lipids become trapped within the membrane and are unable to reach the lipid droplet core.

By precisely modulating the pathways involved in lipid droplet formation and breakdown in yeast, the researchers successfully controlled the size and quantity of intracellular lipid droplets. Their approach to engineering lipid droplet characteristics focused on ten specific genes linked to TAG metabolism and membrane remodeling.
The researchers examined the storage patterns of squalene and zeaxanthin produced inside cells. Their findings revealed that squalene accumulation increased as lipid droplet size expanded. This suggests the following process: when bulky TAGs become diluted by single-chain squalene, the structural integrity of TAG-packed lipid droplets may weaken, facilitating further squalene migration and enabling its substantial buildup in more flexible lipid droplets. The storage capacity for rigid lipids such as zeaxanthin remained largely unchanged in engineered yeast cells, despite an overall increase in lipid droplet surface area. Due to their rigid nature, these lipids were unable to integrate into intracellular lipid droplets and were instead retained within various cellular membranes, including those of organelles and the plasma membrane.
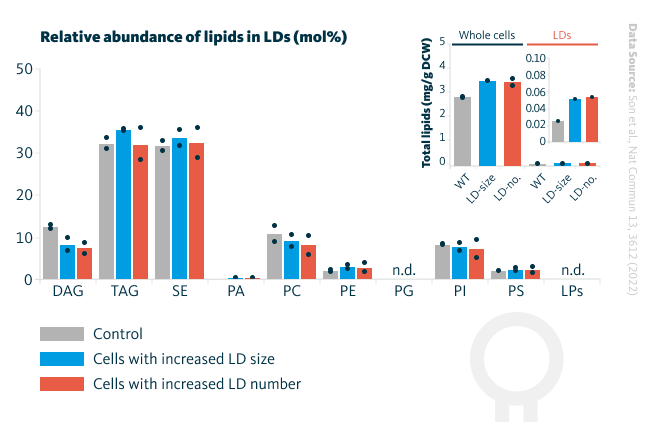
Lipid profiles of lipid droplets in various yeast cells. Lipid profiles of the engineered yeast cells revealed that total lipid and lipid droplet lipid levels in cells with increased lipid droplet size and cells with increased lipid droplet number were slightly elevated compared to control cells. Lipid droplet lipid composition in these cells and total lipid levels were similar. The relative enrichment of lipids in lipid droplets in these engineered cells is illustrated in mol %. DAG, diacylglycerol; TAG, triacylglycerol; SE, sterol ester; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PS, phosphatidylserine; LPs, lysophospholipids; DCW, dry cell weight.
Son et al., Nat Commun 13, 3612 (2022), 10.1038/s41467-022-31400-6
In engineered yeast cells, rigid and non-flexible zeaxanthin exhibited a distinct storage pattern compared to the more flexible squalene. Yeast cells containing numerous small lipid droplets stored more zeaxanthin than those with fewer, larger droplets, indicating that the total surface area of lipid droplets plays a crucial role in zeaxanthin storage. The researchers concluded that the amount of stored zeaxanthin directly correlated with lipid droplet surface area.
Overall, this study characterized different lipid classes within lipid droplets across various yeast cells. The findings suggest that lipid flexibility is a key factor in determining a lipid’s ability to migrate into lipid droplets.
Starvation and lipid metabolism
TANGO2 is a vital protein playing a significant role in essential cellular functions such as ER-to-Golgi transport and mitochondrial health. Mutations in the TANGO2 gene disrupt mitochondrial activity, heighten endoplasmic reticulum stress, and reduce Golgi structure density. These genetic alterations result in early symptoms such as low blood sugar, elevated ammonia levels, muscle degeneration, irregular heart rhythms, and neurological disorders. Over time, these initial effects can lead to cognitive impairments, highlighting the profound impact of TANGO2 mutations on cellular function. Investigating how TANGO2 interacts with cellular pathways offers crucial insights into the underlying causes of several health conditions, like microcephaly or cleft palate.
Agustin Lujan and colleagues examined the precise cellular localization and physiological functions of TANGO2 in cancer cells and primary fibroblasts. Their findings revealed a strong co-localization between the TANGO2 marker and a mitochondrial marker. TANGO2 was predominantly concentrated in regions where mitochondria are closely localized to the endoplasmic reticulum and lipid droplets.
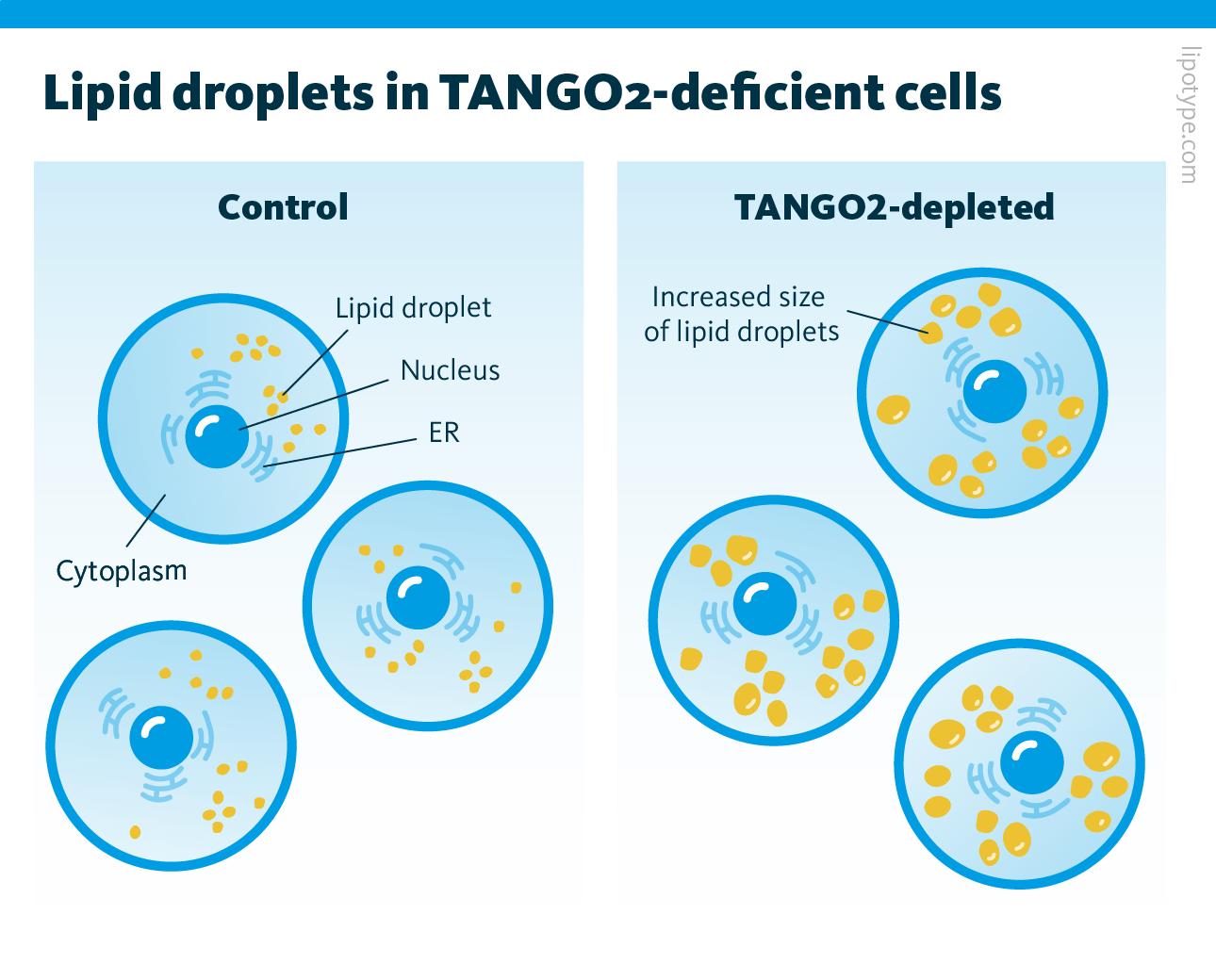
The observation that TANGO2 is often found in regions associated with lipid droplets motivated researchers to explore its role in lipid metabolism and LD regulation in both normal and starved cells. Their findings revealed that the absence of TANGO2 leads to a significant enlargement of lipid droplets, likely due to increased biogenesis and a disruption in lipolysis. Observing these changes in lipid droplet size in TANGO2-deficient cells encouraged the researchers to further investigate alterations in overall cellular lipid composition in TANGO2-deficient cells under both normal and starvation conditions.
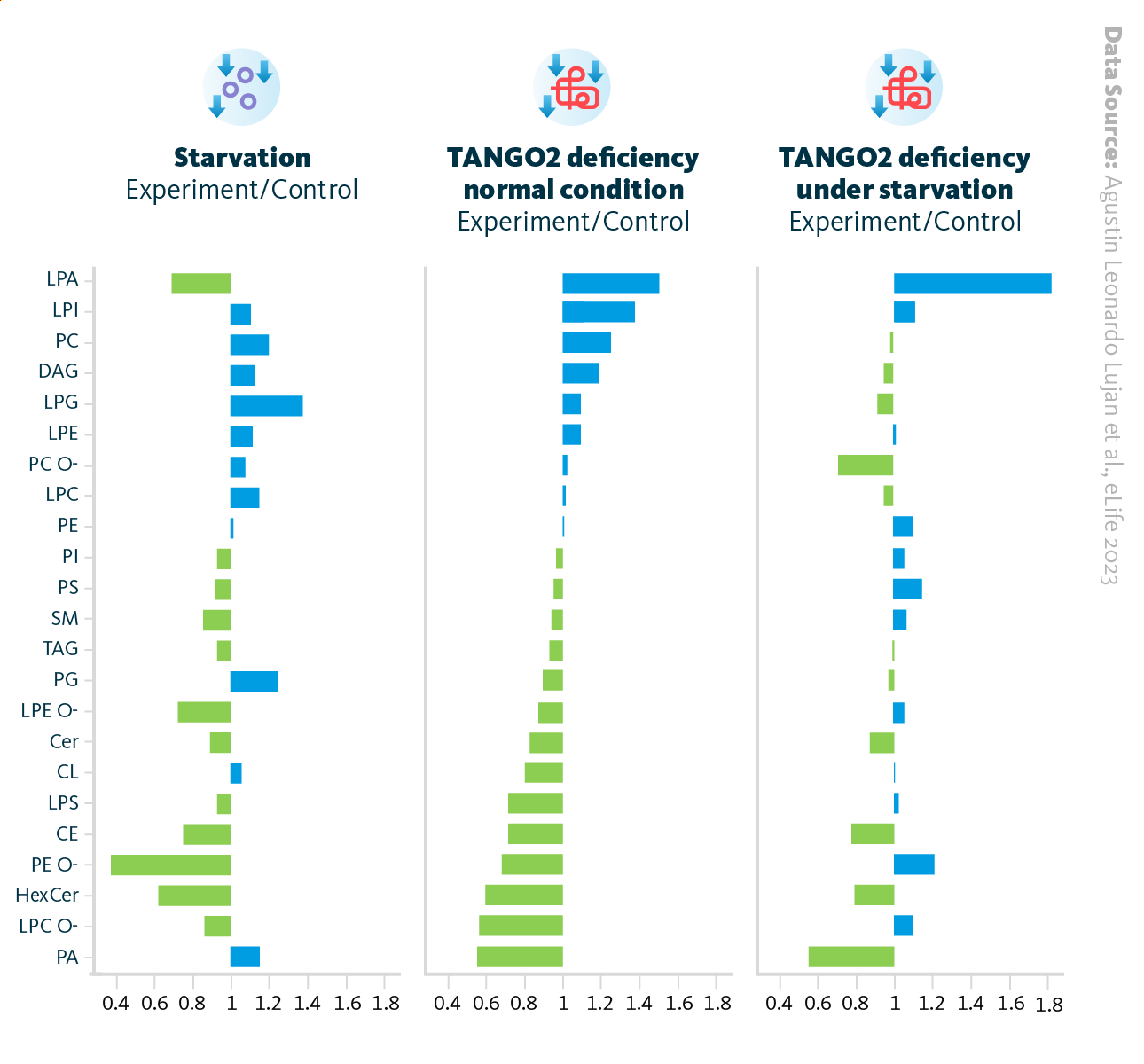
Changes in lipidomics of starved TANGO2-depleted cells. Relative changes in lipid classes under various conditions: nutrient starvation (left), cells with depleted TANGO2 under normal conditions (middle), and cells with depleted TANGO2 during nutrient starvation (right).
Agustin Leonardo Lujan et al., eLife 2023, 12:e85345.; 10.7554/eLife.85345
A detailed lipidomic analysis revealed that TANGO2-deficient cells exhibited elevated levels of lysophosphatidic acid (LPA) and decreased levels of phosphatidic acid (PA), with these lipid profile changes becoming more pronounced during starvation. Additionally, there was a significant reduction in cardiolipin (CL), a lipid typically synthesized from PA in mitochondria. TANGO2 deficiency affects the levels of enzymes involved in both phospholipid and neutral lipid metabolism.
The researchers found that TANGO2 predominantly localizes to mitochondria in mammalian cells, with a partial presence at sites where the ER and LDs closely interact with mitochondria. Knocking down TANGO2 led to a noticeable increase in lipid droplet size and elevated intracellular reactive oxygen species levels, with these effects becoming more pronounced under nutrient-starved conditions. These cellular alterations closely resemble those observed in cells from individuals with TANGO2 mutations. These findings indicate that TANGO2 plays a crucial role in lipid homeostasis, particularly in acyl-CoA metabolism. The study suggests that disruptions in lipid metabolism — worsened under nutrient-starved conditions — are likely key drivers of starvation-induced acute rhabdomyolysis, cardiomyopathy, and cardiac arrhythmias associated with TANGO2 mutations.
Lipids & kidney disease in diabetes
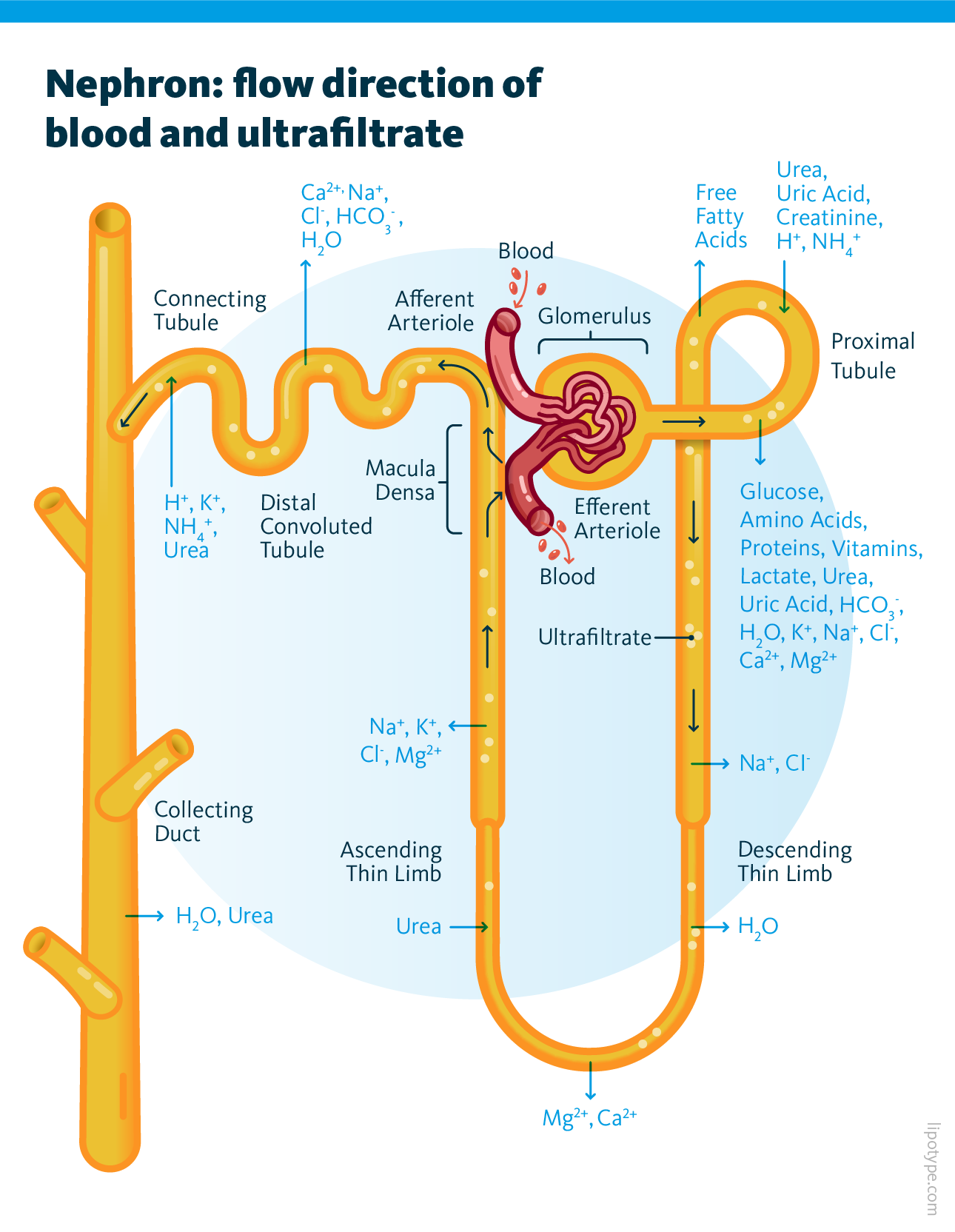
The kidneys are paired organs responsible for several vital functions, including regulating blood water volume by producing urine and eliminating waste and toxins. The nephron, the kidney’s functional unit, operates the following way: first, the glomerulus filters the blood to produce ultrafiltrate, and then the tubule reabsorbs essential substances while removing waste. During this filtration process, the proximal tubule cells (PTCs) reabsorb most solutes, water, glucose, and amino acids. This energy-intensive process primarily relies on fatty acids, which are stored in lipid droplets within segments 2 and 3 of the PTCs. The final result of these processes is urine formation.
In diabetes, nephron damage impairs kidney function, and one major contributing factor is excessive lipid accumulation in lipid droplets, leading to lipotoxicity — one of the key drivers of kidney disease progression. Over time, this damage can result in kidney failure, requiring artificial filtration through dialysis or a kidney transplant.
Scientists investigated lipid metabolism and the impact of accumulating different saturated and monounsaturated free fatty acids in diabetic kidney PTCs. To model diabetes, they administered a low dose of streptozotocin (STZ) in mice, selectively destroying pancreatic islets to induce insulin deficiency, hyperglycemia, and diabetes. The mice were then fed two types of high-fat diets: one enriched in saturated fatty acids (SFA), primarily from butter containing high levels of palmitic acid, and another enriched in monounsaturated fatty acids (MUFA), sourced from olive oil rich in oleic acid. The researchers found that a high-fat diet enriched in SFAs led to significant lipid droplet accumulation in tubules and fibrotic kidney damage in diabetic mice. Although MUFAs accumulated in PTCs more than SFAs, they resulted in significantly less fibrosis.
To further investigate lipid accumulation, the researchers examined whether increased TAG synthesis contributed to lipid droplet formation. By treating renal epithelial cells with different fatty acids, they observed that MUFAs promoted TAG synthesis, leading to the formation of ER-derived lipid droplets.
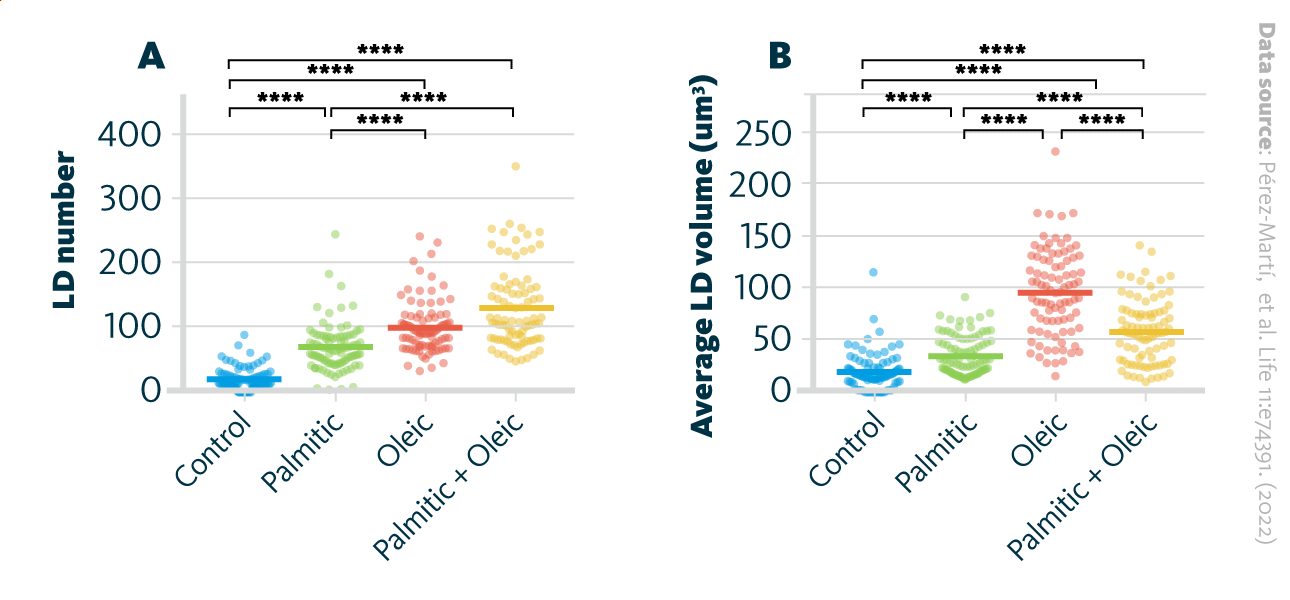
The formation of lipid droplets (LDs) upon treatment with various fatty acids. A LD number and B LD average volume in renal epithelial cells treated for 16 hr with palmitic, oleic, and palmitic + oleic acid. Data are presented as the mean + all values; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; Kruskal–Wallis plus Dunn’s multiple comparisons test; 10 cells per field from three fields were analyzed for three independent biological replicates.
Pérez-Martí, et al. Life 11:e74391. (2022) 10.7554/eLife.74391
Overall, both the mouse model and cell culture experiments investigated disruptions in TAG synthesis as a key driver of lipotoxicity in diabetic kidney disease. The high-fat diet in the mouse experiments and the enriched media in the cell culture experiments demonstrated that oleic acid promoted TAG production, whereas palmitic acid increased diacylglycerol (DAG). saturation and impaired TAG synthesis. Collectively, these findings highlight the protective role of TAG-enriched lipid droplet formation and provide deeper insights into the mechanisms underlying lipotoxicity in diabetes.
Lipid metabolism impacts remyelination
Remyelination is a regenerative process that follows damage to the central nervous system (CNS). This process often fails in conditions such as multiple sclerosis and Alzheimer’s disease. Microglia, a specialized type of glial cells, serve as the brain and spinal cord’s innate immune cells. They play a crucial role in the inflammatory response and in facilitating myelin repair after demyelination. A deeper understanding of remyelination and lipid composition remodeling is critical for developing interventions aimed at reconstructing the myelin sheath. Following CNS injury, myelin debris accumulates from the breakdown of the myelin sheath wrapped around axons. This debris consists of fragmented myelin membranes, cellular remnants, and lipids, including cholesterol, phospholipids, ceramides, and other sphingolipids.
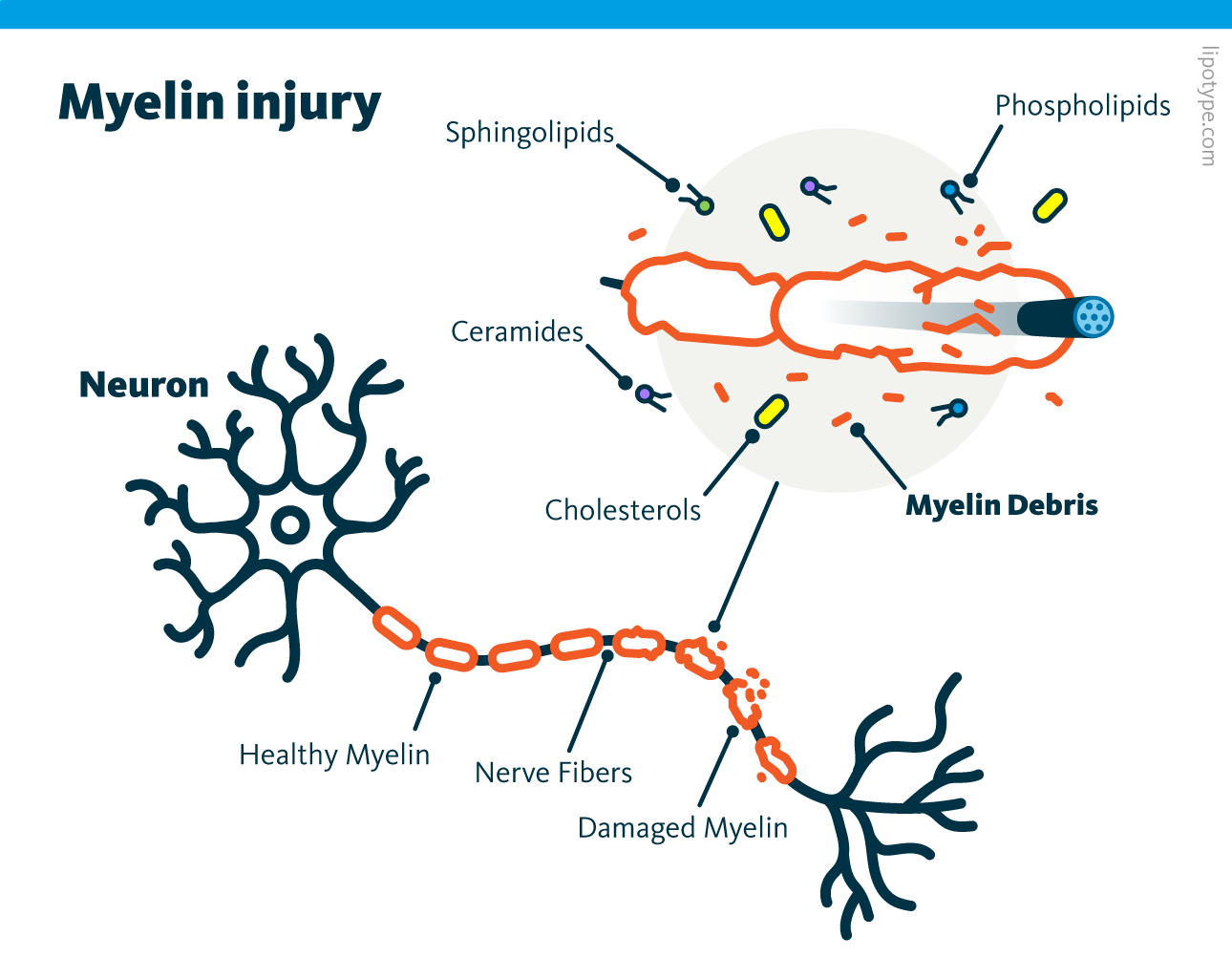
Normally, cholesterol cannot be degraded by lysosomal enzymes during the clearance of myelin debris and is transported from late endosomes to the ER. The ER has a limited capacity to process excess free cholesterol. To prevent toxicity, free cholesterol is esterified into cholesteryl esters and stored in lipid droplets. Proper cholesterol exposure is essential for efficient myelin debris uptake. When this lipid droplet buffering mechanism is impaired, microglial phagocytes fail to resolve demyelinating lesions, leading to interruptions in the regenerative response required for successful remyelination.
Gouna and Simons investigated the role of triggering receptor expressed on myeloid cells 2 (TREM2) in the remyelination metabolic pathways, particularly the one facilitating myelin debris clearance. The research was performed in cell cultures and mouse brain and spinal tissues. It was revealed that TREM2 deficiency disrupts the adaptive response to excess cholesterol exposure. It is likely that TREM2-dependent gene expression is required for the synthesis of enzymes involved in lipid droplet formation, a process crucial for effective remyelination.
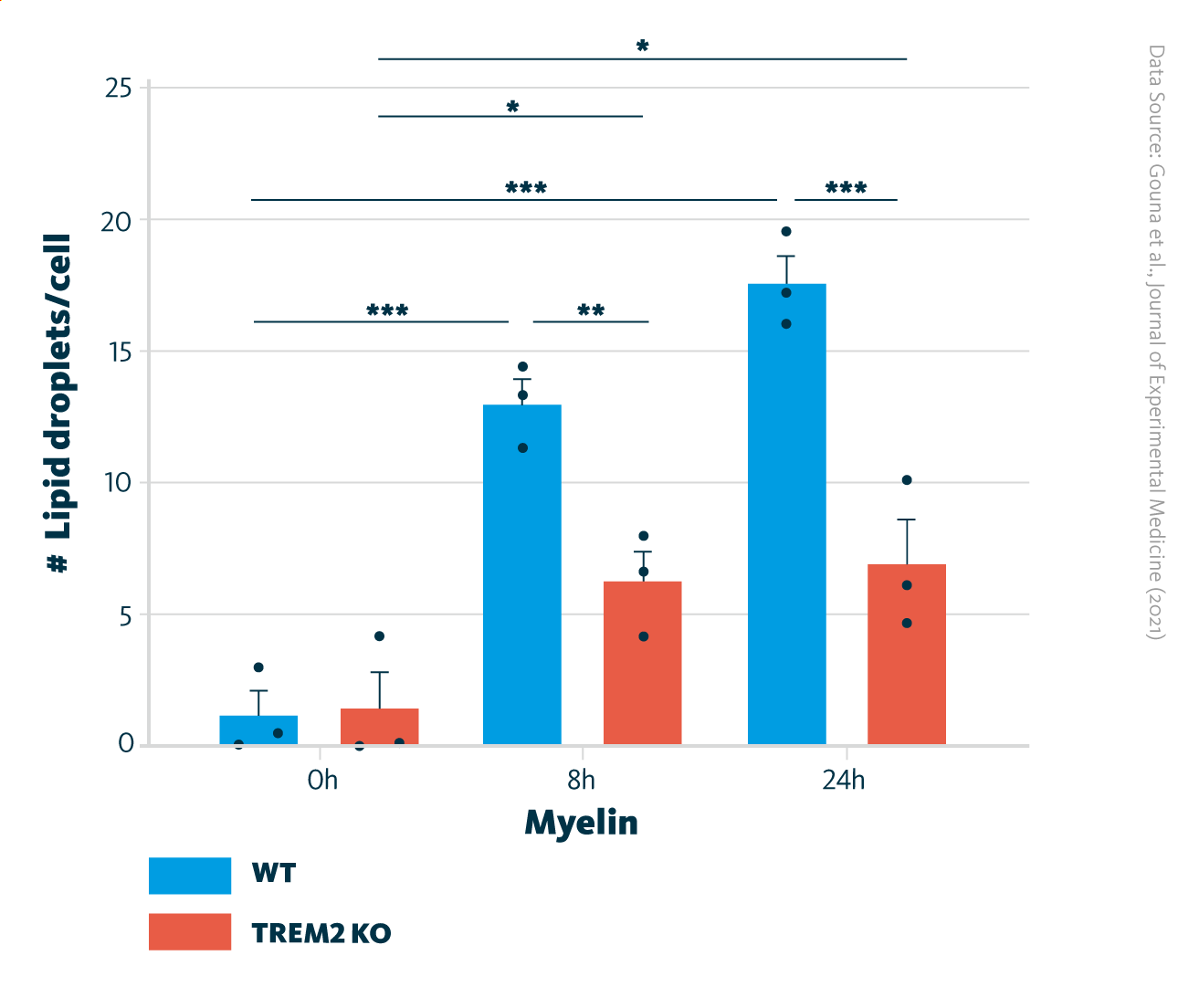
Comparison of lipid droplet formation in TREM2-KO and WT cells. Quantification of the number of lipid droplets per cell in postnatal microglia. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Gouna et al., Journal of Experimental Medicine (2021), doi:10.1084/jem.20210227
Lipid analysis showed that TREM2-deficient phagocytes failed to generate cholesteryl esters and had impaired TAG synthesis. The increase in phosphatidic acid abundance in these cells suggests the reduced activity of lipin that catalyzes the conversion of phosphatidic acid to diacylglycerol.
Although TREM2-deficient phagocytes can internalize myelin debris, they fail to initiate the necessary metabolic responses for effective remyelination. As a result, these cells accumulate toxic levels of free cholesterol over time. This leads to cholesterol-induced cellular stress, and the authors observed clear signs of ER stress in myelin debris–loaded TREM2-deficient phagocytes.
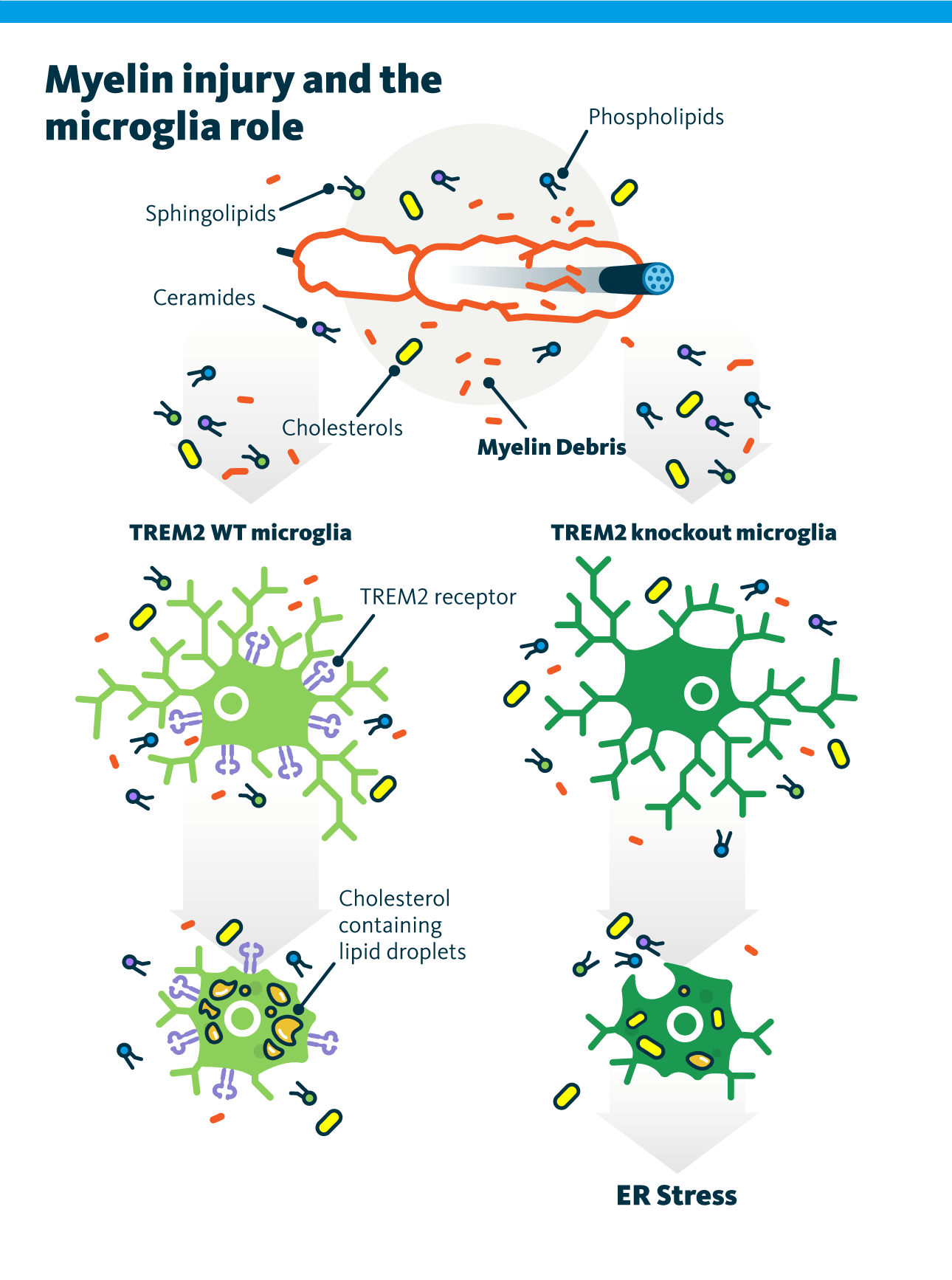
This study examined the role of lipid droplets in phagocytes in clearing excess cholesterol that accumulates following a demyelinating injury. In summary, efficient cholesterol efflux and esterification are crucial for resolving innate immune inflammation and facilitating remyelination.
The role of lipid droplets in ischemic stroke
In neurological conditions, lipid droplet accumulation appears to drive microglia into a dysfunctional, pro-inflammatory state. Lipid-droplet-rich microglia (LDRM) has been linked to worsened outcomes in aging brains, neurodegenerative diseases, and cerebral circulation disorders like ischemic stroke.
This study explores the relationship between lipid metabolism, microglial phenotypes, and inflammation in ischemic stroke models to learn the mechanisms underlying stroke-induced neuroinflammation. In both in vitro and in vivo models, microglia exposed to hypoxia and inflammatory stimuli exhibited a significant increase in lipid droplet formation and altered lipid metabolism.
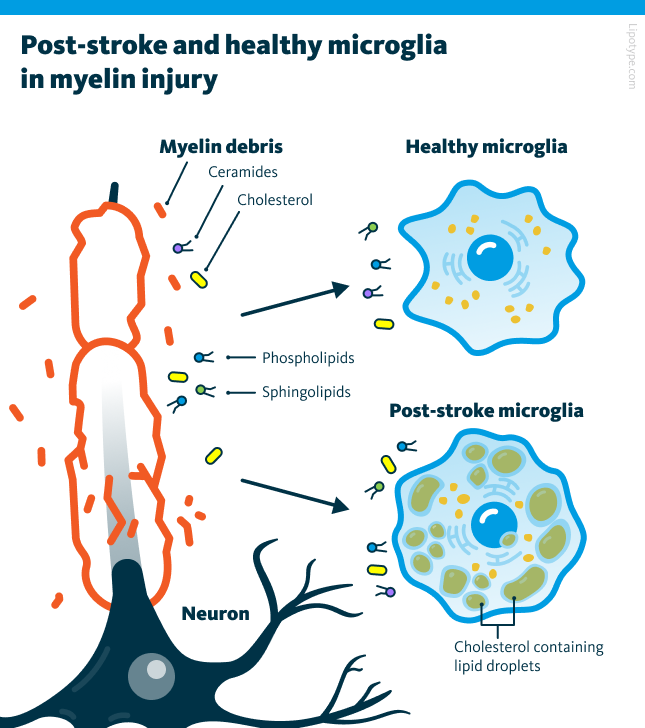
In vitro experiments with primary microglia in hypoxic or inflammatory conditions demonstrated significant lipid droplet accumulation. Inhibiting Acyl-CoA acyltransferase (ACAT) substantially reduced lipid droplet accumulation, indicating that lipid metabolism plays a critical role in regulating microglial activation in ischemic conditions.
An in vivo mouse model of middle cerebral artery occlusion (MCAO) was used by the researchers to investigate lipid profiles in the ischemic core and surrounding regions. Lipidomics analysis was conducted on tissue samples from various brain regions at different time points following stroke. Lipidomic analysis revealed distinct lipid changes in the brain regions affected by ischemic stroke, with the ischemic core showing a significant increase in cholesteryl esters, TAG, and DAG. Dynamic alterations in phosphatidylcholine (PC), phosphatidylethanolamine (PE), and their ether-linked forms (PC O-, PE O-) were observed during the early stages after stroke. These suggest that localized lipid changes may directly influence microglial activation and inflammatory responses following ischemic injury.
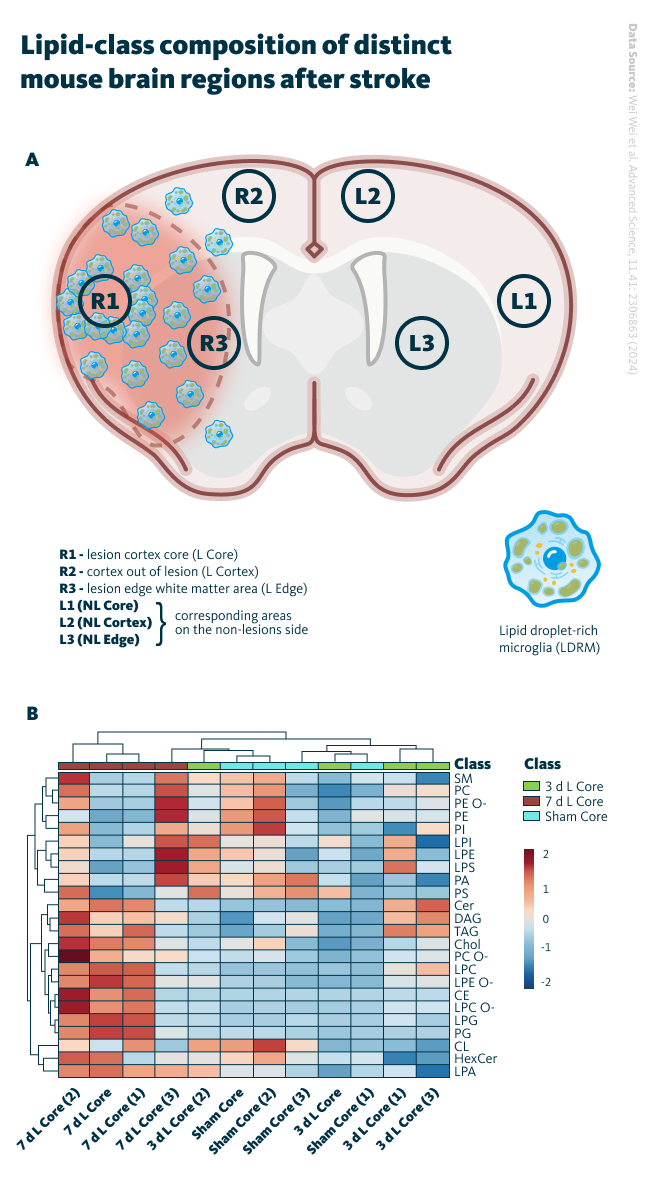
Lipid-class composition of distinct mouse brain regions after stroke. A Brain regions of interest in MCAO mouse model for lipidomics. Definition of regions of interest on MCAO-induced mouse brain tissue; (here the definitions of Rs and Ls). B Heatmap of core regions representing color-coded Z scores of lipid-class distributions of the 24 most abundant lipid classes using the lipid species concentrations (pmol) with log2 normalization.
Wei Wei et al. Advanced Science, 11.41: 2306863 (2024), 10.1002/advs.202306863
Lipid alterations in the ischemic brain appear to be closely connected to microglial activation and the accumulation of lipid droplets. The core region of the affected brain tissue showed a strong association between lipid composition and pro-inflammatory microglia, whereas the surrounding areas exhibited a more anti-inflammatory profile, accompanied by lower lipid accumulation. These findings suggest that microglial function and lipid metabolism are interdependent, and variations in lipid composition may directly influence the immune response and extent of neural damage following ischemia.
This research highlights the critical role of lipid droplets during ischemic stroke. The results indicate that ischemia-driven lipid changes shape microglial activity and inflammation. The ability of microglia to transition between pro- and anti-inflammatory states is largely influenced by the composition of lipids in their environment. A deeper understanding of these metabolic shifts may support the discovery of novel treatments aimed at restoring microglial balance and resolving neuroinflammation across various brain disorders.
How to use lipidomics in lipid droplet research?
Lipidomics helps study lipid droplets by analyzing their composition, metabolism, and role in health and disease. It tracks lipid changes during metabolic shifts, supporting research on metabolic and neurological disease, and more. It also can be used to identify disease biomarkers and evaluate therapeutic effects, offering insights for potential treatments.
Lipotype Lipidomics Technology is a powerful tool for analyzing lipid droplets. By providing detailed data on lipid composition and abundance, lipidomics enables researchers to study how lipid droplet profiles change in response to various physiological conditions, drug treatments, or environmental factors.
Do you have any questions?
We can answer them!