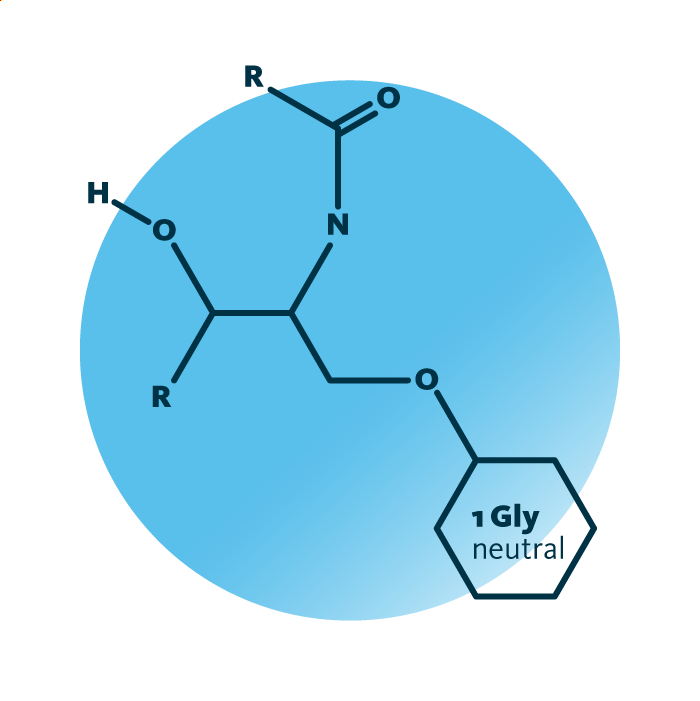
Structure. Cerebrosides belong to the sphingolipids. Their structure consists of a ceramide backbone linked to a neutral/non-acidic monosaccharide “head” group, such as galactose or glucose. The ceramide backbone of cerebrosides contains a hydrocarbon chain termed long-chain base; one fatty acid is linked to the ceramide.
Function. Cerebrosides are present in bacteria and eukaryotes such as animals, plants, and fungi. Many cerebrosides serve as precursors to diglycosylceramides and thus also to glycosphingolipids with more than two sugars in the head group (e.g. globosides, gangliosides, and sulfoglycosphingolipids). Apart from their biosynthetic role, they serve important biological functions.
Galactosylceramides are among the most researched cerebrosides and essential to myelin, the insulating layer that wraps around nerves. Glucosylceramides are an important constituent of the skin to maintain trans-epidermal water loss and may support the cell membrane of plants to overcome cold and drought stress. Little is known about cerebrosides with more exotic sugar molecules.