THE endoplasmic reticulum (ER) is a cellular organelle that is mainly responsible for the folding of newly synthesized protein molecules and transporting these properly folded proteins in vesicles to the Golgi apparatus. The rough endoplasmic reticulum is largely involved in protein synthesis, while smooth endoplasmic reticulum is generally used for the synthesis and storage of lipids.
ER stress is a chronic disturbance process that impacts the ER metabolism and homeostasis. Researchers started to investigate ER stress in the year 1968 and more and more research has been done in this field each year. The number of publications increased from one paper in 1968 to over 3000 by the end of December 2022.
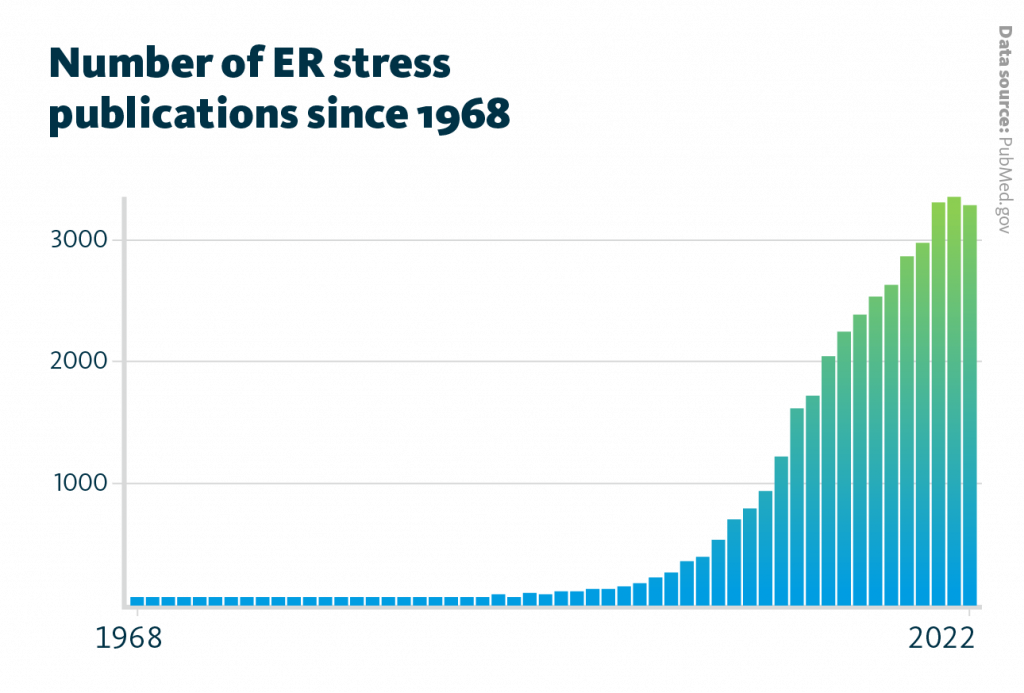
Number of ER stress publications since 1968: The number of annual publications which mention ER stress began to continuously increase from about 100 publications in 2000 to over 3,000 publications by the end of 2022.
PubMed.gov
ER stress is characterized by the accumulation of abnormal proteins, leading to the disbalance of the protein folding capacity and slowing its speed. Since only correctly folded proteins are transported to the Golgi apparatus, unfolded proteins accumulate in ER and cause ER stress. Microenvironmental conditions such as hypoxia, cell-intrinsic metabolic alterations, oncogenic stress, and certain medical interventions can lead to ER stress.
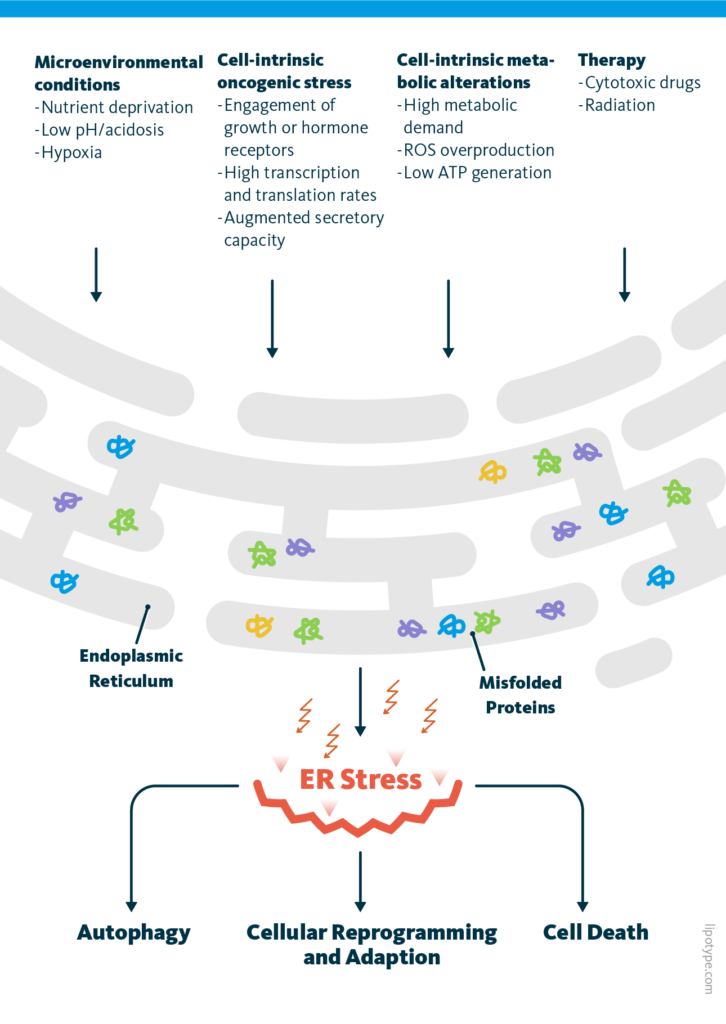
The unfolded protein response (UPR) is a cellular response to ER stress, that evolved to protect the cell from ER stress. Upon activation, the UPR aims to restore normal cell function by pausing protein synthesis, degrading misfolded proteins, and activating processes to support protein folding. If these goals are not achieved within a certain time span, the ER stress triggers the cells to undergo apoptosis or, best case scenario, cellular reprogramming, and adaptation.
ER stress can activate three different intracellular signal transduction pathways: protein kinase RNA‐like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol‐requiring enzyme 1 alpha (IRE1α); they are collectively termed the unfolded protein response. Certain changes in lipid metabolism can trigger UPR, for example, phospholipid metabolism aberrations in the ER or the exposure to saturated fatty acids activate the IRE1α pathway.
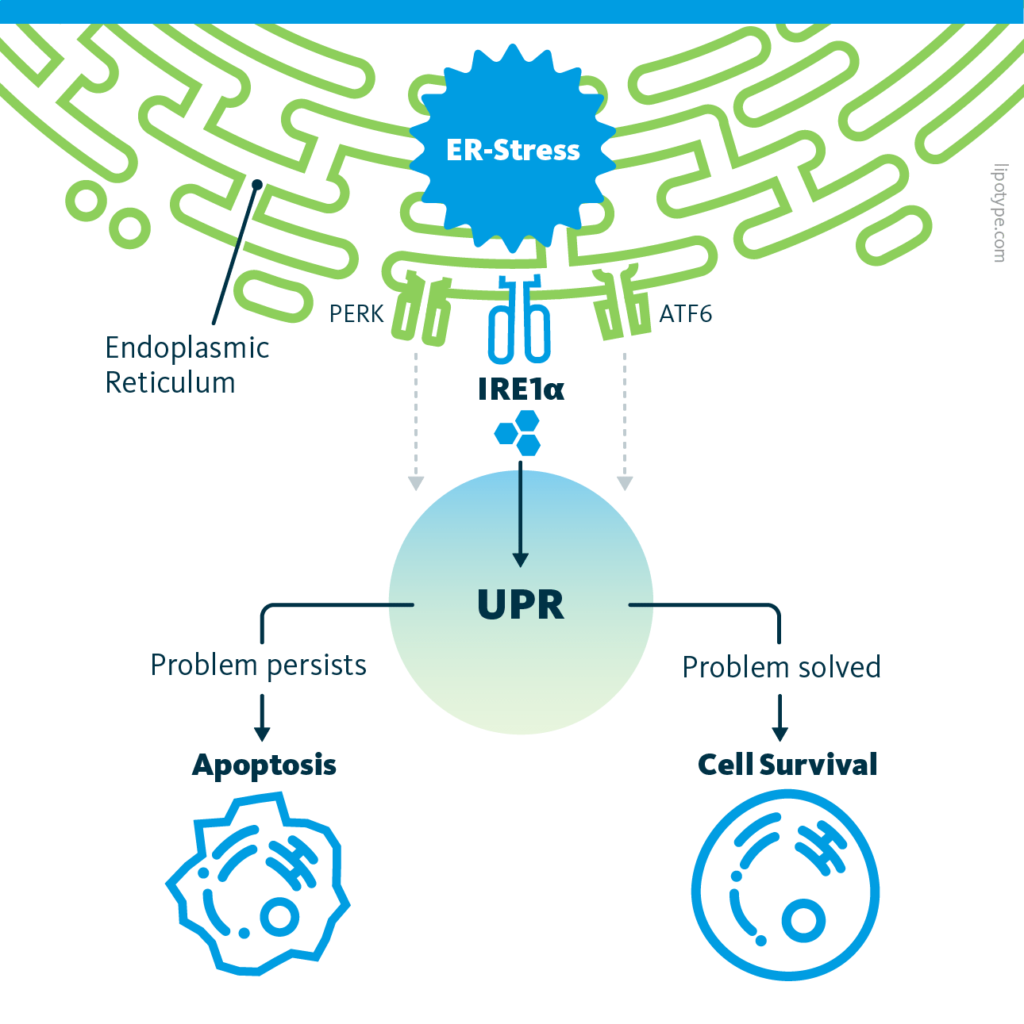
The effect of UPR on lipid metabolism depends on the UPR activation mechanism. Briefly, IRE1α signaling affects lipolysis, triacylglycerol (TAG) synthesis, fatty acid (FA) elongation and desaturation, and lipogenesis. The PERK pathway also affects FA elongation and desaturation, lipogenesis and, additionally, mevalonate pathway. ATF6 also affects lipogenesis, mevalonate pathway and, finally, FA oxidation. ER stress is emerging as a cause of drastic lipid metabolism changes, that leads to disturbance of processes like remyelination or immune response to vaccines, and disease development, for example, breast cancer and diabetic kidney disease.
ER stress and breast cancer
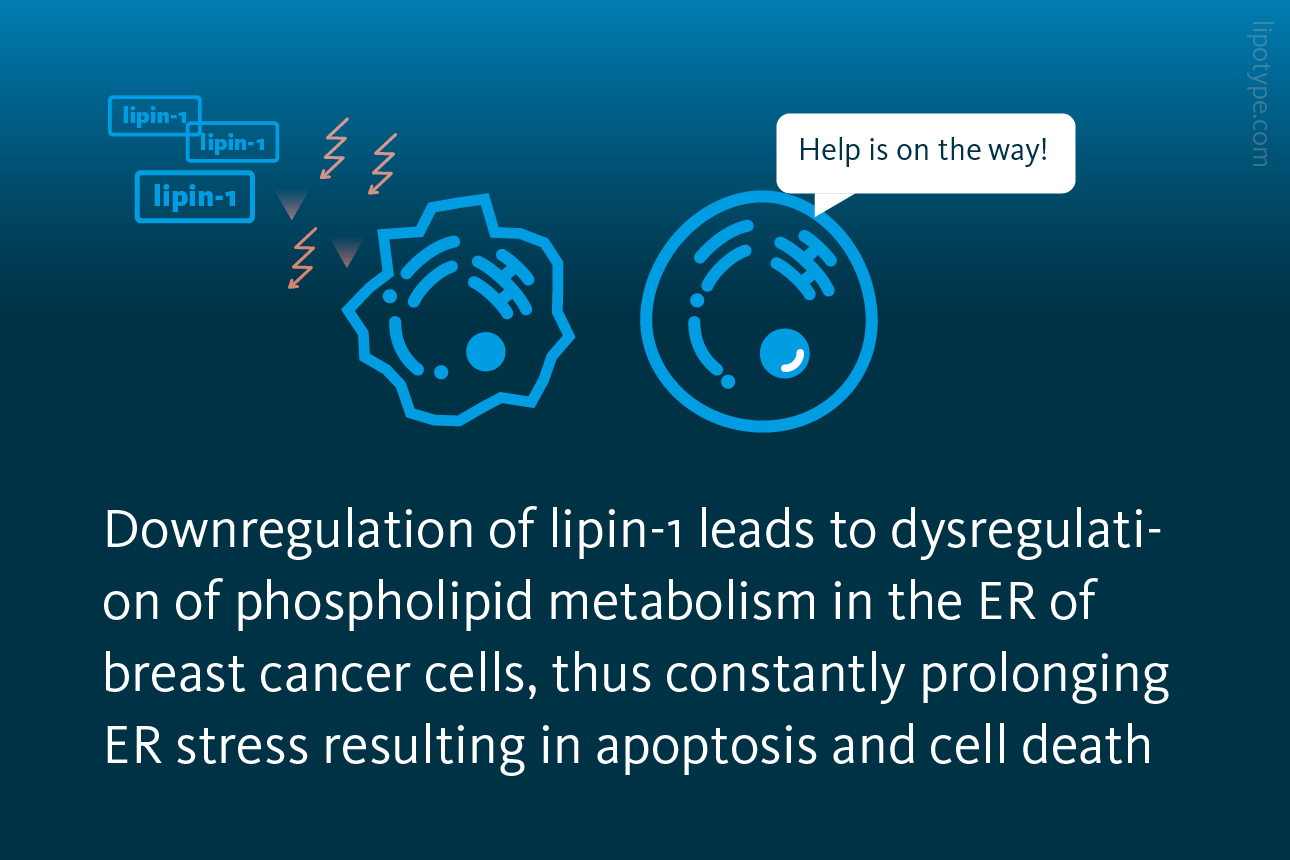
ER stress can also be used to the benefit of health. One example is the utilization of ER stress in triple negative breast cancer (TNBC). In this context, the gene LPIN1, responsible for producing the enzyme lipin-1, experiences a significant upregulation. The overexpression of LPIN1 is strongly associated with decreased patient survival rates. To shed light on the function of lipin-1 in breast cancer phospholipid metabolism and its relationship with ER stress, researchers by down-regulated lipin-1 production in triple-negative breast cancer cells.
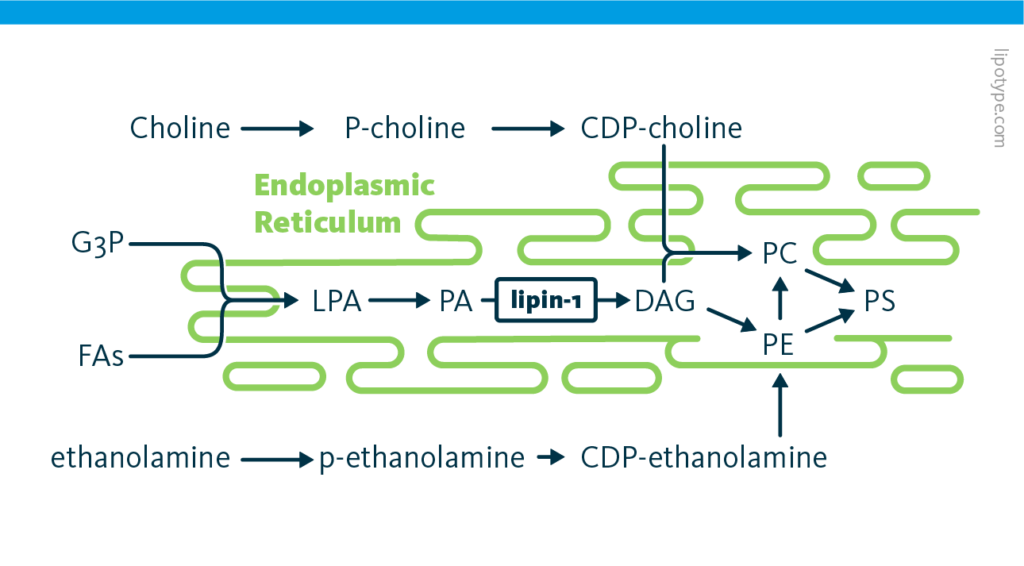
General pathway for phospholipid synthesis: The biochemical pathways of phospholipid synthesis in mammalian cells. The enzyme lipin-1 is highlighted.
He et al., FASEB Journal (2017), doi: 10.1096/fj.201601353R
Down-regulating lipin-1 in TNBC cells resulted in a disruption of phospholipid metabolism within the ER. Consequently, this led to persistent activation of the IRE1α signaling pathway and the UPR. Ultimately, the prolonged ER stress induced apoptosis, leading to the death of the TNBC cells.
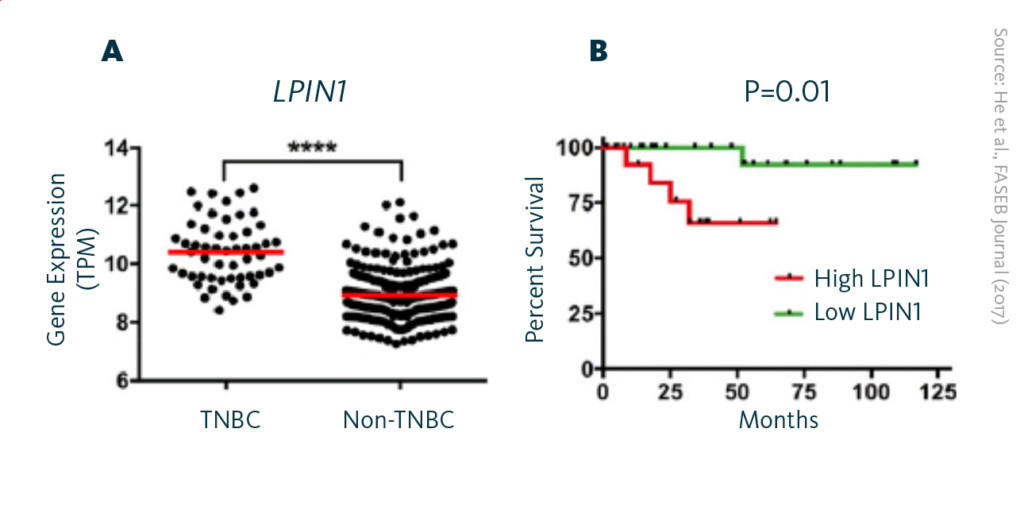
LPIN1 expression in TNBC: A Comparison of the LPIN1 gene readout levels in TNBC and non-TNBC breast cancers. B High LPIN1 gene readout correlates with shorter overall survival of patients with TNBC.
He et al., FASEB Journal (2017), doi: 10.1096/fj.201601353R
The significance of lipin-1 in maintaining endoplasmic reticulum (ER) homeostasis and promoting tumor growth in TNBC was further validated in a mouse model. The findings confirmed the critical role of lipin-1 in TNBC progression, even in an in vivo setting. This evidence highlights the potential therapeutic strategy of targeting phospholipid metabolism as a means to prolong ER stress. Such an approach can help patients with TNBC and potentially in other types of cancer as well.
ER stress, lipid droplets, and remyelination
One of the processes ER stress is involved in is lipid droplet formation during the remyelination process in demyelinating diseases like multiple sclerosis.
During the normal process of myelin debris clearance, cholesterol, unlike other lipids, cannot undergo degradation by lysosomal enzymes. Instead, it is transported from late endosomes to the ER. However, the ER is unable to handle the excessive accumulation of free cholesterol. To address this, free cholesterol is converted into cholesteryl esters through esterification and stored within lipid droplets.
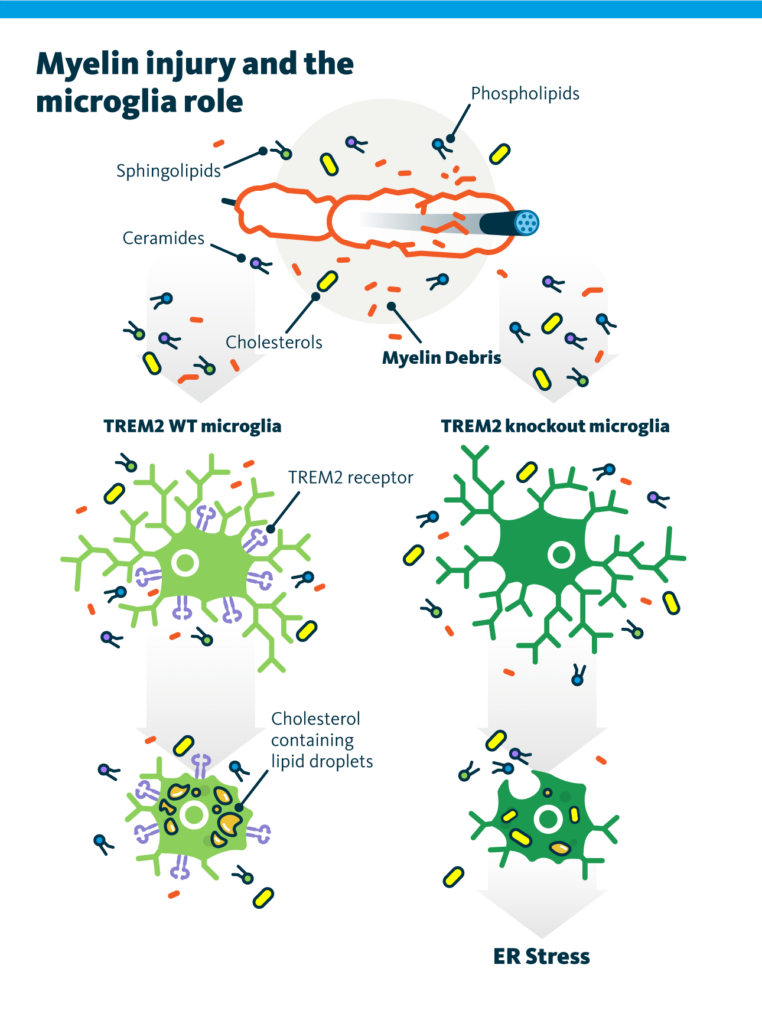
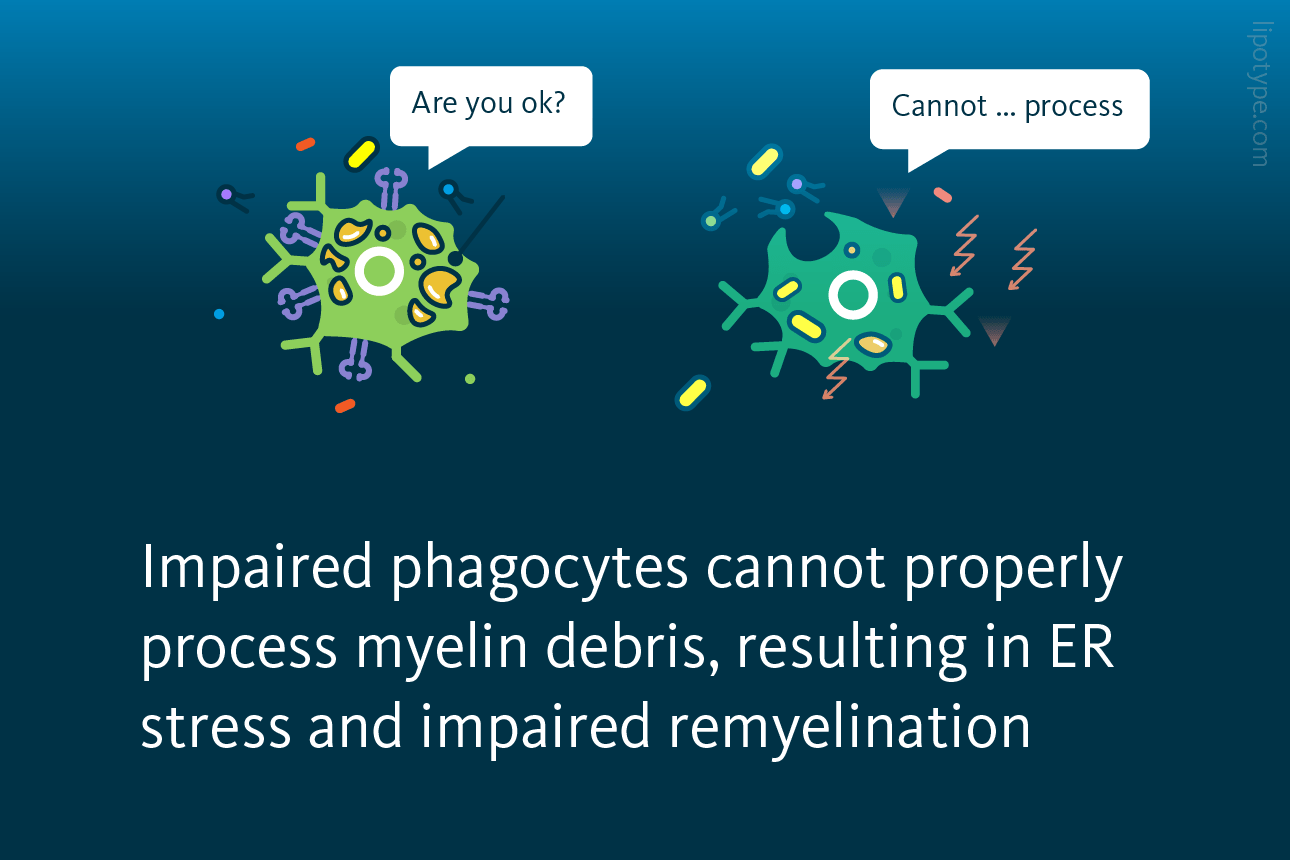
Proper uptake of myelin debris by microglia macrophages requires exposure to cholesterol. This cholesterol serves as a crucial component in facilitating the clearance process. In cases where the buffering mechanism of lipid droplet formation is impaired, microglia phagocytes fail to effectively resolve demyelinating lesions. Consequently, the regenerative response during remyelination is compromised.
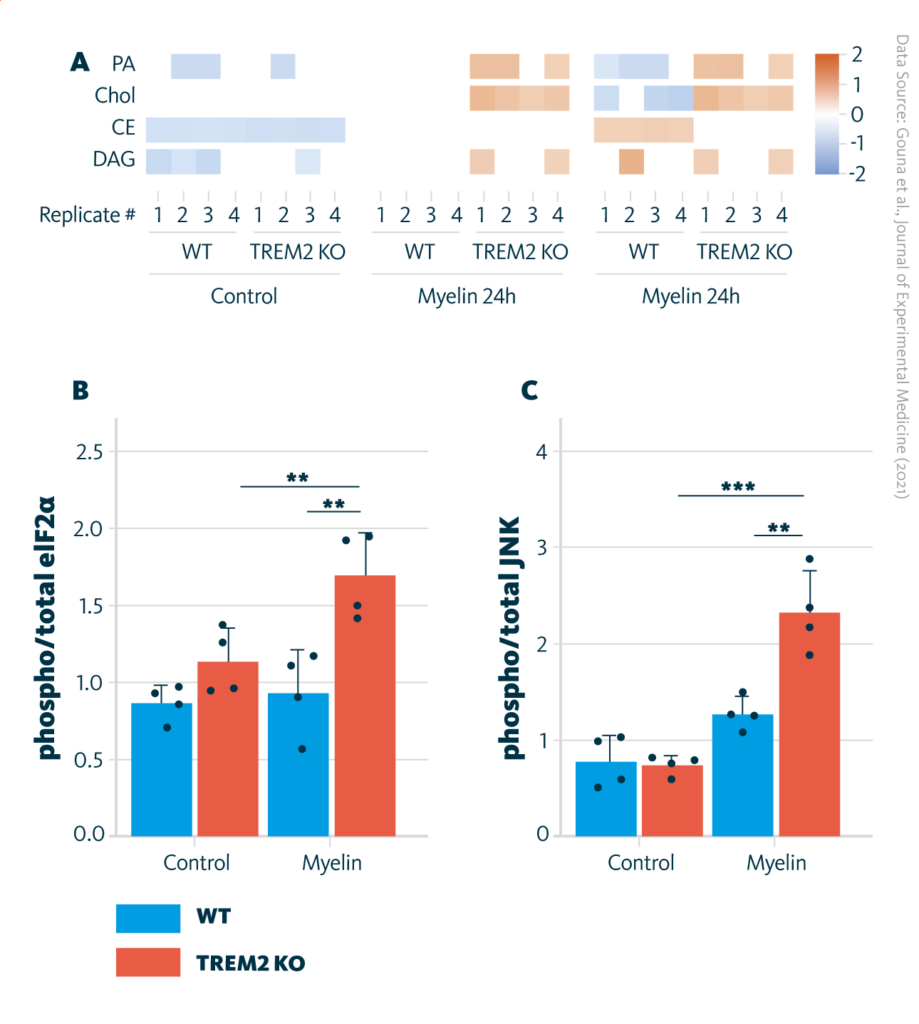
Comparison of lipid profiles in TREM2-KO and WT cells and ER stress presence: A Untargeted lipidomics analysis of WT and TREM2 KO cells cultured in serum-free media or with myelin for 8 or 24 h. B and C Analysis of ER stress by quantification of phosphorylated eIF2α (B) and phosphorylated JNK (C), measured by Western blot. **, P < 0.01; ***, P < 0.001.
Gouna et al., Journal of Experimental Medicine (2021), doi:10.1084/jem.20210227
Phagocytes with the impaired myelin debris uptake are able to internalize myelin debris but fail to process it and become exposed to the lipotoxicity of free cholesterol that builds up with time. Consequently, cholesterol-induced cellular stress develops, and the evidence of ER stress in myelin debris–loaded impaired phagocytes is observed. This leads to cells’ inability to resolve innate immune inflammation and to support remyelination.
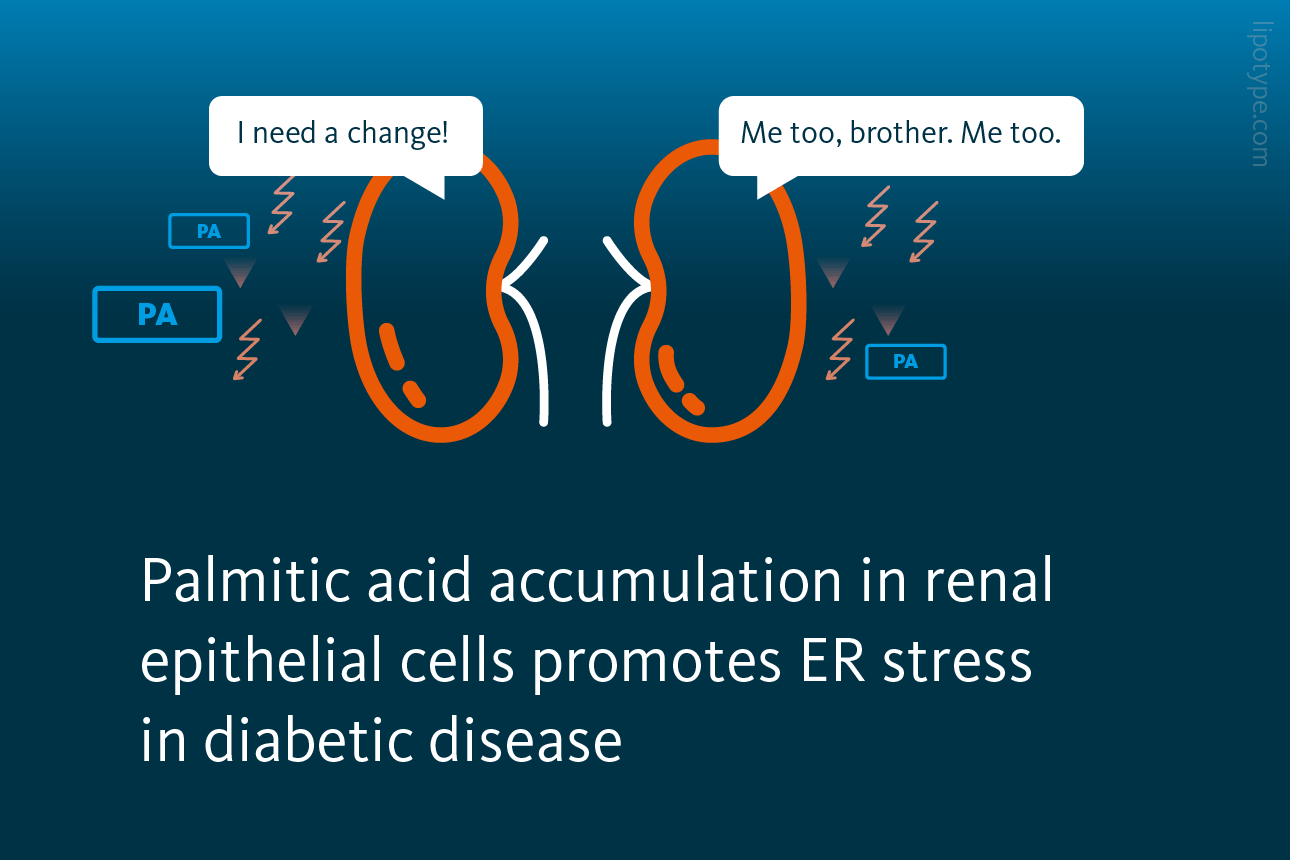
ER stress, diabetes, and kidney disease
The functioning of the nephron involves two main steps. Firstly, the glomerulus filters the blood, resulting in the formation of ultrafiltrate. Subsequently, the tubule carries out the vital task of returning necessary substances back to the blood while removing waste products. In the process of blood filtration, the majority of solutes, water, glucose, and amino acids are reabsorbed within the cells of the proximal tubule (PTC). Some of these processes may be affected in case the kidney gets damaged during diabetes development; ER stress plays a crucial role in it.
One of the ways kidney functional units can get damaged during diabetes is due to excessive accumulation of lipids leading to lipotoxicity which is one of main drivers of kidney disease progression.
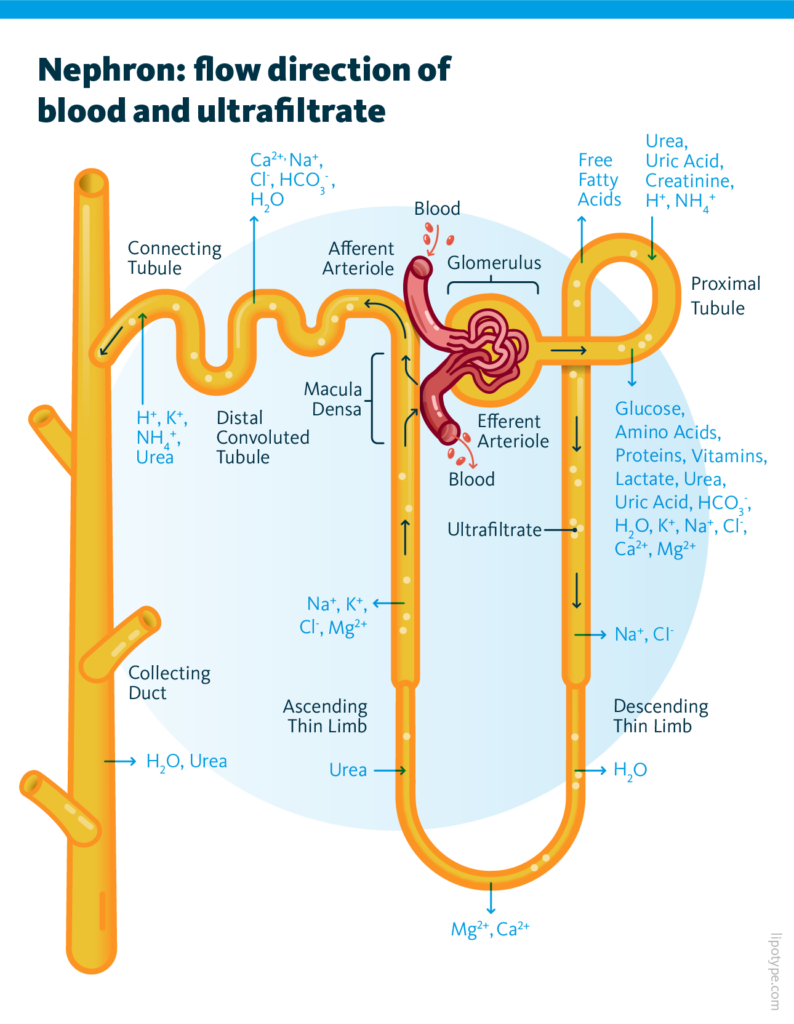
In particular ER stress advances in response to dietary palmitic acid accumulation in renal epithelial cells. ER stress can be activated by several different pathways, and all ER stress response pathways are activated by dietary palmitic acid. Adding oleic acid to the cell media reversed the process and rescued the cells from ER stress. Interestingly, the protective effects of oleic acid are specific for palmitic acid-induced ER lipid bilayer stress.
The researchers investigated whether elevated synthesis of triglycerides (TAG) results in the accumulation of lipids within lipid droplets. They examined the number and volume of lipid droplets in renal epithelial cells exposed to different fatty acids. The findings revealed that mono-unsaturated fatty acids promoted the formation of triacylglycerols, leading to the subsequent generation of lipid droplets derived from the endoplasmic reticulum.
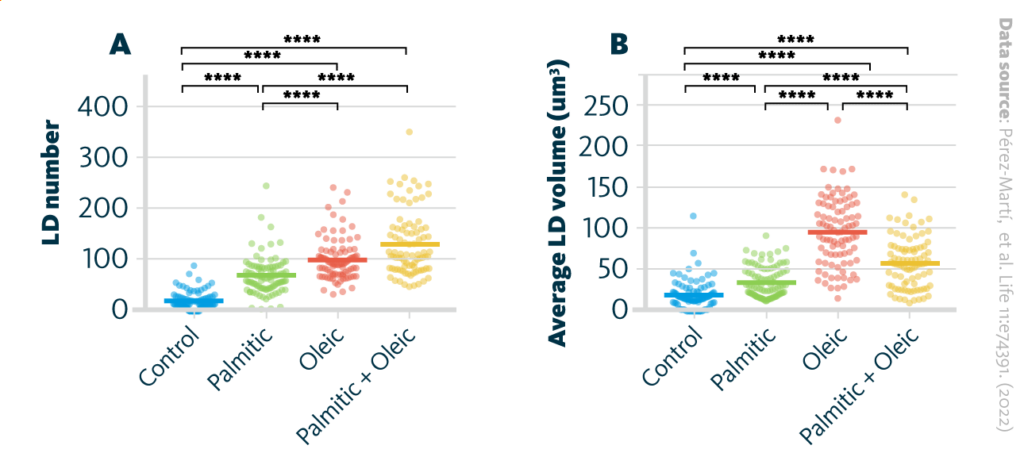
The formation of lipid droplets (LDs) upon treatment with various fatty acids: A LD number and B LD average volume in renal epithelial cells treated for 16 hr with palmitic, oleic, and palmitic + oleic acid. Data are presented as the mean + all values; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; Kruskal–Wallis plus Dunn’s multiple comparisons test; 10 cells per field from three fields were analyzed for three independent biological replicates.
Pérez-Martí, et al. Life 11:e74391. (2022) 10.7554/eLife.74391
Lipid metabolism-wise, the oleic acid treatment led to the strong formation of triacylglycerols, and palmitic acid treatment led to increased levels of precursors of triacylglycerols, such as diacylglycerols, phosphatidic acid, and lysophosphatidic acid. The addition of oleic to palmitic acids enhanced the formation of triacylglycerols, leading to reduced accumulation of diacylglycerols, phosphatidic acid, and lysophosphatidic acid. Oleic acid also decreased the concentration of free fatty acids detected in cells, most likely due to stimulated triacylglycerol synthesis.
These results once again showed that short fatty acids cause ER stress and impair triacylglycerol production in cultured cells. Overall, the set of mice and cell culture experiments evaluated the disturbances in triacylglycerol synthesis as a key mechanism driving lipotoxicity in diabetic kidney disease.
ER stress and adjuvants in vaccines
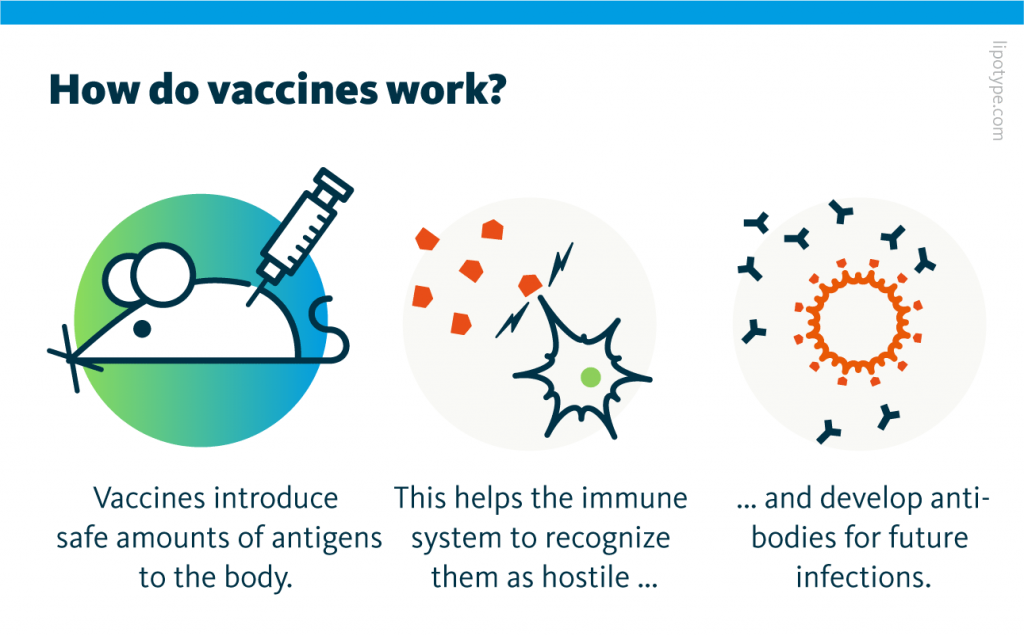
Vaccines play a crucial role in training the immune system to recognize and combat pathogens. They achieve this by introducing antigens into the body, which in turn stimulate an immune response. In modern vaccine development, adjuvants are utilized to amplify the effect of antigens, eliciting a stronger immune reaction while requiring fewer antigens. Adjuvants serve as a vital component in ensuring that even tiny doses of pathogens can trigger a robust and durable immune response within the body.
Lipid-based adjuvants play an important role in the efficiency of vaccines. By applying lipidomics, researchers studied a specific lipid-based adjuvant AS03’s effect on lipid metabolism and changes in lipid composition triggered by the adjuvant in a mouse model organism. It was discovered that the lipid metabolism remodeling initiated by AS03 leads to ER stress, thus activating IRE1α and resulting in an upregulation of genes related to inflammation.
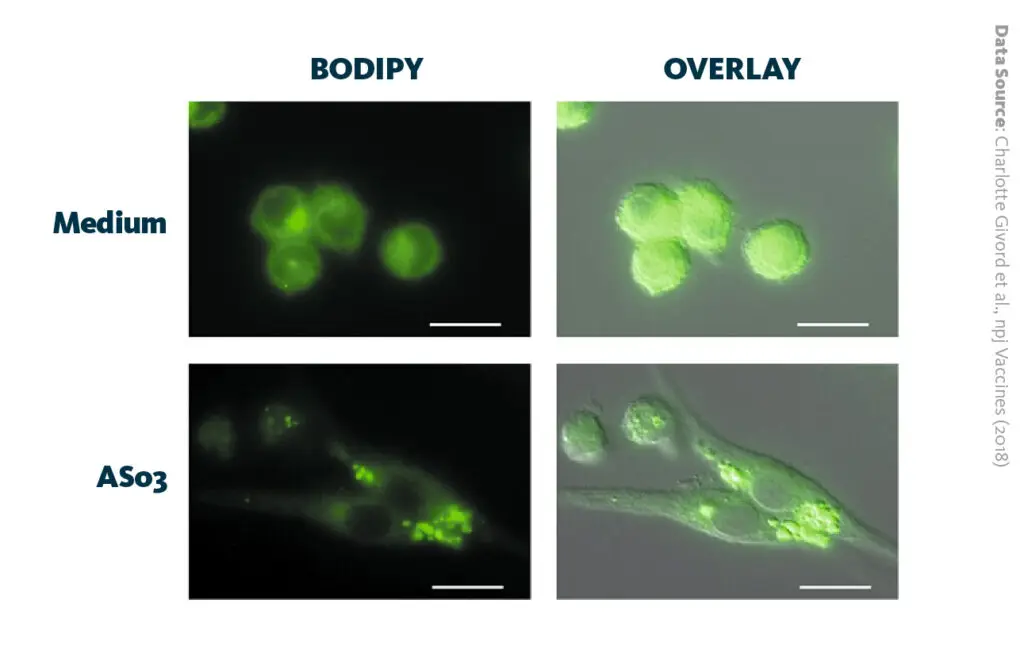
AS03 induces the rapid formation of lipid droplets in macrophages: Activation of the endoplasmic reticulum stress sensor IRE1α by the vaccine adjuvant AS03 contributes to its immunostimulatory properties.
Givord et al., npj Vaccines (2018), 10.1038/s41541-018-0058-4
The lipidomics analysis demonstrated that AS03 affects cholesterol and fatty acid metabolism of macrophage cells in lymph nodes, showing a decrease of cholesterol but an increase of phosphatidylcholine lipids, a class of phospholipids which is mainly synthesized in the endoplasmic reticulum.
Ultimately, this increases cytokine production and induces a protective immune response, the reason for the immuno-stimulatory properties of the lipid-based adjuvant AS03. These findings pave the way for the development of new lipid-based adjuvants in vaccines.
How to use Lipidomics to understand ER stress
Quantitative lipidomics analysis allows not only to detect the changes in lipid metabolism triggering ER stress and UPR, but also to identify of the subsequent changes in lipid metabolism in a case when the ER stress resolves in autophagy, apoptosis, or cell reprogramming. Pinpointing these changes is essential to get deep insights into relevant diseases’ development and progression, thus allowing scientists to work on treatment and prevention as well.
Lipotype Lipidomics technology can be used to characterize the lipidomics changes triggered by the ER stress in patient samples, tissue from model organisms, and in vitro samples. These data can provide insight into a wide variety of diseases, including neurodegenerative diseases, various cancers, and metabolic diseases.
Do you have any questions?
We can answer them!